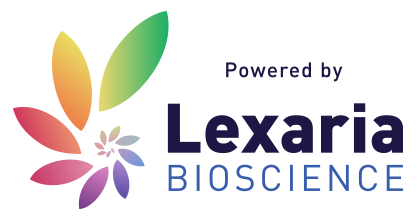Technology


Commercial Applications
Lexaria’s DehydraTECH is generally used for formulating and delivering lipophilic (i.e., fat-soluble) drugs and active ingredients. DehydraTECH is protected by a robust suite of pending patent applications or patents already granted around the world that cover its use with a broad range of bioactive molecules.
Lexaria’s technology is best thought of as an additional layer that improves the effectiveness of existing or planned new products for companies that offer prescription and non-prescription-based drugs or supplement type products. Lexaria licenses its advanced technology to other companies around the world to offer consumers the best performance possible across an array of product formats.
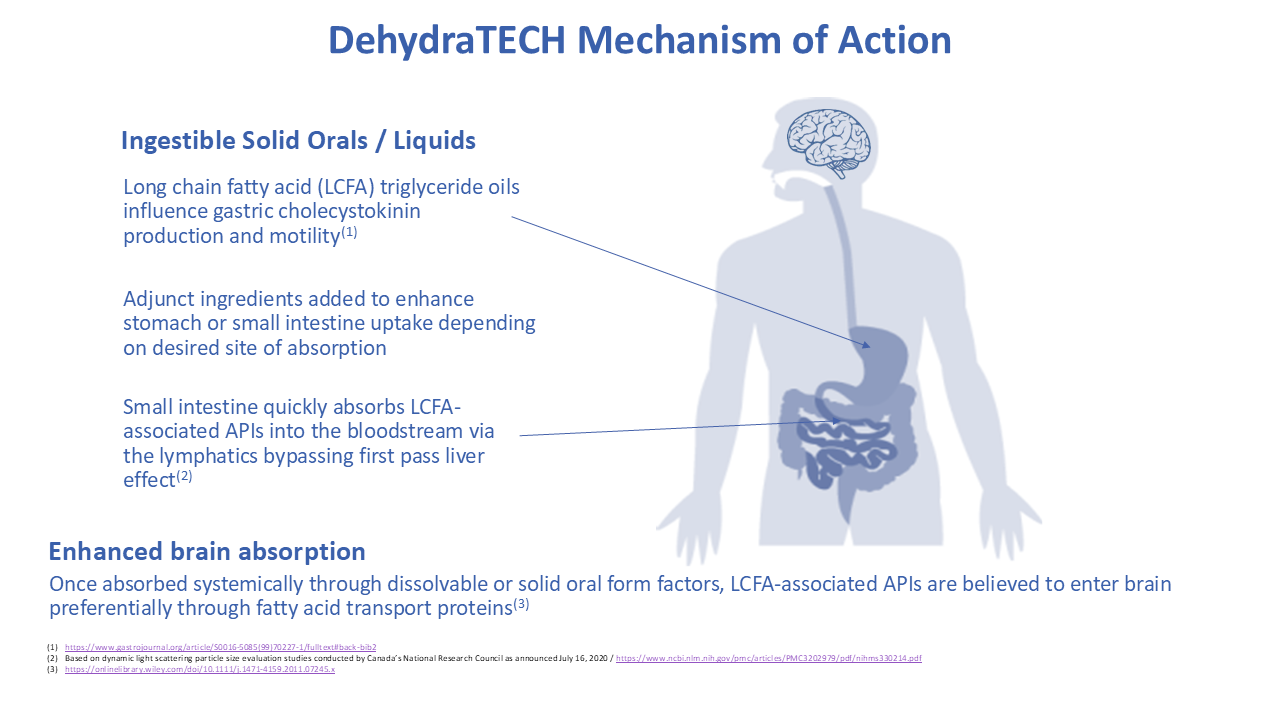
Lexaria’s ongoing R&D is mainly focused on development of product candidates across various key segments.
Pharmaceuticals: GLP-1 Drugs
GLP-1 drugs have been approved by the FDA for type two diabetes, weight loss management, and most recently in 2025 for sleep apnea, chronic kidney disease, and noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) in the liver.
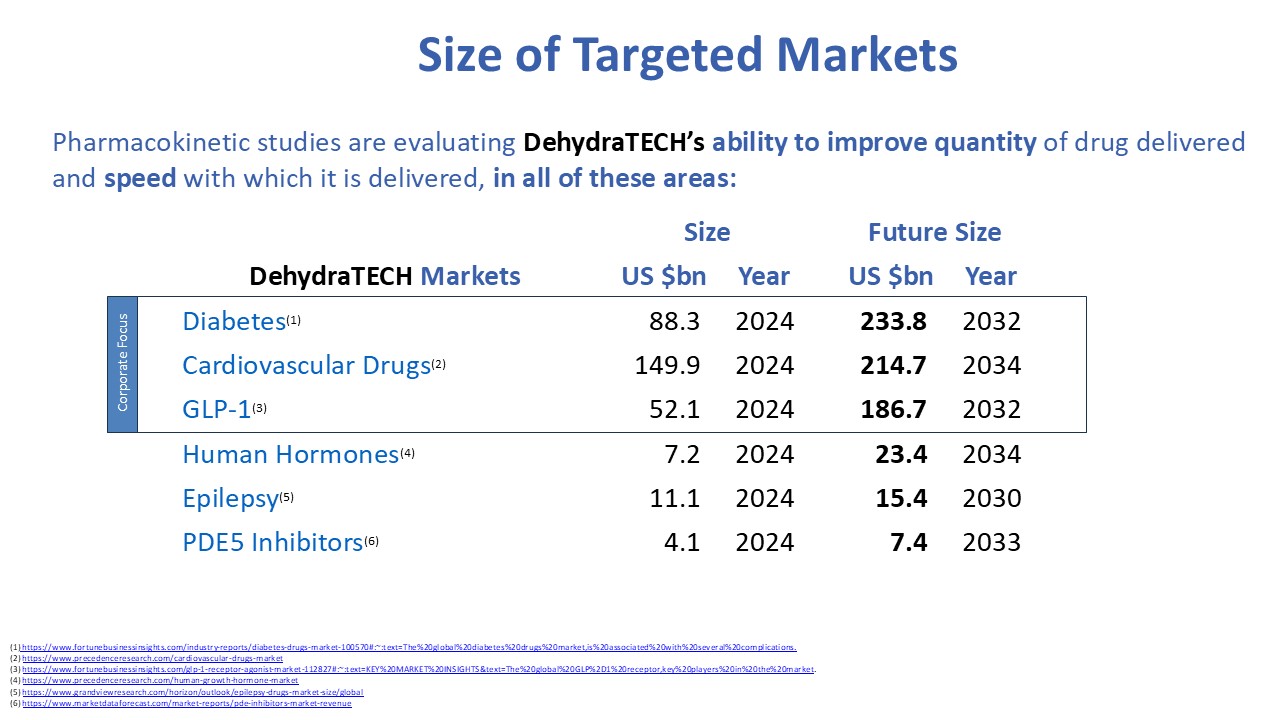
Anecdotal commentary also suggests that some patients are experiencing reduced cravings for alcohol, nicotine and opioids while taking GLP-1 drugs. Other trials are examining their effects on heart disease and even dementia in part because of evidence that GLP-1 drugs may reduce the build-up of the proteins amyloid and tau in the brain, thought to be partly responsible for Alzheimer’s disease. Because the health benefits of this drug class are still being discovered and understood, the potential market size is unknown. Reports are estimating $186.7 billion in sales per year, by 2032.
Side effects of GLP-1 drugs vary but can include nausea, vomiting, diarrhea and more. A small number of GLP-1 drugs have already been tested or approved in oral format but some studies have reported worse side effects with the oral form. The drugs are also being investigated for their relationship to bone density, muscle loss and more. Because of potential serious side effects, it may be beneficial to treat patients with lower oral doses of the drugs, something that Lexaria’s DehydraTECH technology may enable if it can improve the PK performance of GLP-1 drugs through oral capsules.
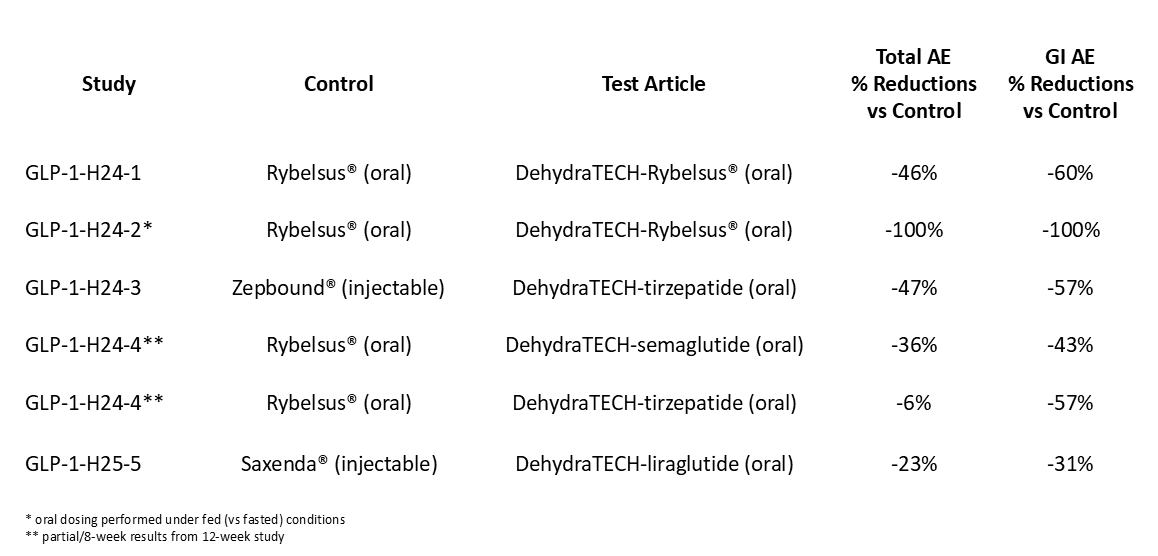
To that end, Lexaria is working to position its DehydraTECH drug delivery technology as not only an improved drug absorption/performance enhancer, but also as a vital enabler of reducing side effects of various GLP-1 drugs. The following table summarizes the dramatic improvements in GI AEs that Lexaria has realized when testing all three (semaglutide, tirzepatide, and liraglutide) of the major GLP-1 drugs on the market today with its DehydraTECH (“DHT”) technology in an oral format:
Pharmaceuticals: Hypertension and Heart Disease
The current global market size for drugs used to treat various heart diseases is $149.9, and anticoagulants, antihypertensive, and antihyperlipidemic drugs comprise roughly 90% of these. The market is expected to reach $214.7 billion by 2034.
Cardiovascular diseases are the leading cause of death globally, taking an estimated $17.9 million lives each year, and in the U.S., nearly one-half of all Americans suffer from some form of cardiovascular disease. Lexaria has already demonstrated in a pilot human clinical study, the ability to statistically significantly decrease human blood pressure, theorized to have occurred as a result of the known vasodilatory potential of our substance of choice. The generic version is known to have poor absorption characteristics, such that typically only about 6% of what is orally ingested actually finds its way into blood circulation. Research has shown that Lexaria’s processing technology increases absorption by 100% to 500%. Lexaria’s DehydraTECH is currently patented in the European Union and Australia for treatment of heart disease.
Pharmaceuticals: Epilepsy
Lexaria has conducted studies in rodents utilizing DehydraTECH processed CBD whereby it was determined that our technology’s enhancement of CBD mitigated epileptic seizures in rodents at lower doses and more rapidly than the commercially available cannabinoid-based anti-seizure medication, Epidiolex®. Based on these findings, Lexaria has filed patent applications for Compositions and Methods for Treating Epilepsy of which seven US patents and one Australian patent have been granted to date.
The global epilepsy market size was estimated at US $11.1 billion in 2024. The broader anti-convulsant market, which includes epilepsy, is estimated at $25.8 billion in 2024. The American Journal of Managed Care has estimated the total annual costs per person with epilepsy to be US$15,414.
Pharmaceuticals: Other Pharmaceutical Areas of Focus
Our Lexaria Pharmaceutical Corp. subsidiary has worldwide rights to our patent portfolio related to pharmaceutical applications, including a broad range of other active molecules such as antiviral drugs, hormone treatments utilizing estrogen or testosterone, treatments for dementia, rheumatoid disease, and phosphodiesterase inhibitors (PDE5).
Lexaria’s technology significantly enhances oral delivery of certain antiviral drugs. To date we have evidenced that DehydraTECH improves the delivery of each of the following drugs into animal bloodstreams: darunavir, efavirenz, remdesivir, ebastine, and cholchicine; all of which are known to possess various antiviral properties.
Meanwhile, an in vitro screening assay determined that DehydraTECH-enabled remdesevir and ebastine were effective in inhibiting the COVID-19 SARS-CoV-2 virus.
Viral-caused diseases such as HIV, hepatitis, influenza, and coronavirus remain common and at times reach pandemic proportions. Hepatitis causes 1.3 million deaths each year; 40 million cases of influenza were reported in the 2023-2024 flu season in the US alone. Additionally, there are currently 40.8 million people worldwide infected with HIV/AIDS.
According to market research, the global pharma industry was worth $1.7 trillion in 2024, and the ten largest pharmaceutical R&D budgets for 2023 accounted for more than $125 billion. This is a larger R&D expenditure than the entire gross revenues of many other business sectors. Lexaria is pursuing or investigating multiple pharmaceutical related applications of DehydraTECH with bioactive molecules that act upon human CB1 and CB2 receptors. Substances that act upon these receptors in the human body that affect pain, inflammation, anxiety and depression have been shown to have effect against cancer and neurodegenerative conditions.